
When selecting inorganic compound materials, there are critical differentiating factors to consider; among these are cost, purity, packing and particle density, as well as phase and porosity.
A quality finished product is achieved by several combined factors. It requires starting out with high purity source material as well as having excellent reaction and manufacturing processes in place. It is possible to begin with a high purity material, but if the reaction process and/or the manufacturing processes are sub-sufficient, the end product will be less than desirable. A poor manufacturing process will affect melting behavior, particle size distribution and process stability.
Here we will trace the significant developments of yttrium fluoride (YF3), a mature, critical low index material used in infrared (IR) and ultraviolet (UV) thin film engineering. For some industries, such as IR coatings, the cost of ultra high purity materials is prohibitive. For such applications, a high purity material that has not been optimized for the entire spectrum is sufficient, providing the manufacturing processes are excellent.
Materion’s industry expertise and broad manufacturing capabilities allows us to satisfy a range of yttrium fluoride requirements for a variety of markets. Our chemical engineers have created a material that exhibits the best attributes throughout the total YF3 transmissive spectrum (spanning the UV –VIS and IR range). It is able to meet more demanding applications, such as those for UV coatings that require low oxygen, high purity, and completely reacted yttrium fluoride, in order to yield superior quality thin films.
While this may sound fundamental, the reaction process for high purity YF3, as well as the design and application of consolidation processes, is highly complicated. Conventional wisdom states that there are two possible ways to produce yttrium fluoride: (1) either through the reaction of oxide, or the use of hydroxide with hydrofluoric acid, as shown in the following equation:
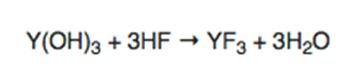
Note that water is a by-product of this reaction. Since YF3 has low water solubility, engineering processes can be employed that will control or eliminate water prior to melting. Without going into the detailed chemistry, focus on the fact that the purity of the product is dominated by a number of factors: the quality of the hydroxide precursor, the acid, and the isolation of the product from the vessel and subsequent consolidation processes. It is accepted that yttrium oxide will have a similar reaction.
While this is perhaps the most obvious technique for yttrium fluoride production, there are additional considerations that pose challenges to chemists. In the below process, the prevalence of heat and side reactions is understated. Yet these oxide reactions can cause complications in varying degrees, if only due to the number of steps necessary to control the reactions safely. To visualize the concept, review the following set of reactions (2) which compete for relevance inside a reaction chamber:
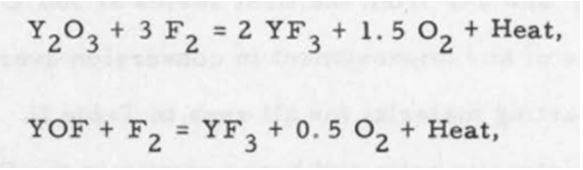
This particular reaction can produce a very dry material but there are other factors that need to be addressed. Running a gas/solid reaction is strongly dependent on surface area (products and reactants) which determine heat generation, by-product formation and pressure increase.
The YF3 reaction process must also be controlled in order to minimize possible side reactions and ensure a completely reacted product. Optimizing heat and pressure is paramount for safe, large-scale, homogeneous powder production.
As you can see, with either method a high degree of complexity occurs long before the product is ready to press or melt into the classic evaporation material form. The attention to detail that is exercised in the reaction process creates a material that is optimized for the manufacturing processes.
Materion has invested in various melting/consolidation technologies to create superior YF3 forms. Our 50+ years of advanced chemicals experience enable us to produce high quality materials with customizable particle sizes and purities. With our new higher purity YF3, we can meet even the most demanding challenges of the thin film and lighting industries.
References:
(1) http://en.wikipedia.org/wiki/Yttrium(III)_fluoride
(2) Manifacier, JC, J Gasiot and JP Fillard, “A Simple Method for the Determination of the Optical Constants n, k and the Thickness of a Weakly Absorbing Thin Film,” Journal of Physics E: Scientific Instruments, 1976, V9, p. 1003.