Fine silver has the highest electrical conductivity (over 100% IACS) of all the electrical contact materials. Silver does not readily oxidize, although it does have a tendency to form sulfide and chloride films. Its hardness and wear resistance may be improved by alloying it with other contact metals such as copper, palladium, or platinum.
Silver-based composite materials such as silver-nickel and silver metal oxides offer significantly improved resistance to arc erosion, as compared to fine silver. Silver can most often be found in the form of contact buttons staked or welded to the blades of electrical switching contacts. Often, a high performance base metal such as C17200 or C17460 is used in the blades to provide and reliably maintain the appropriate contact force. Meanwhile the silver or silver-based contact button provides a high conductivity, arcing-resistant, wear resistant interface.
Because silver has the highest electrical and thermal conductivities of all the contact materials, it is ideal for handling high electrical currents. Fine silver is defined as having greater than 99.9% purity. It has only moderate wear resistance, with a hardness of only 75-200 HV, similar to soft gold. Despite this lack of durability, silver contacts should be mated with a wiping action. This will disrupt the sulfide and chloride films which tend to form on the surface.
Silver-copper alloys with a minimum of 92.5% silver are known as sterling silver. Coin silver is 90% silver, 10% copper. These alloys are harder and less electrically conductive than fine silver, in proportion to the copper content. Their corrosion resistance is similar to fine silver, hence the use of sterling silver in eating utensils. However, these alloys will readily form sulfide and chloride films and the copper component can make them susceptible to oxidation. The tendency to oxidize increases in proportion to the copper content.
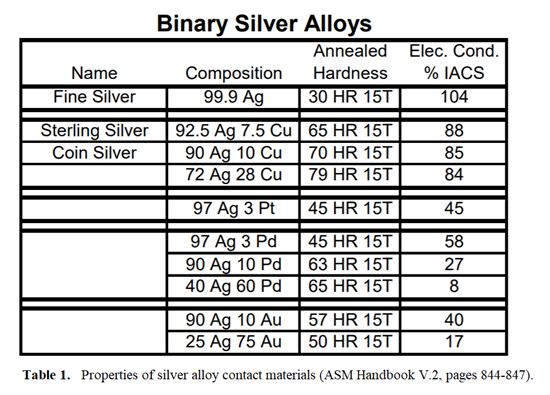
Platinum, palladium and gold are also commonly added to silver to improve its corrosion resistance and increase its hardness. These alloys, however, will show lower conductivity than fine silver. Very small amounts of nickel also may be added to these alloys to improve the hardness without appreciably decreasing the conductivity. Table 1 shows how these alloying elements affect the hardness and conductivity of silver (data taken from ASM Handbook Volume 2). These are just a few of the silver alloys available, but this table shows in a general way how the alloying elements affect silver’s hardness and conductivity.
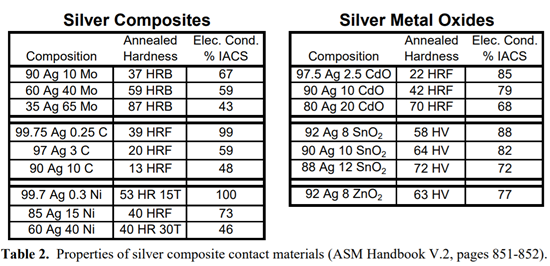
Silver composites are usually produced through powder metallurgy processes, since the constituents are not mutually soluble and cannot be alloyed. These include silver metal oxides (silver-cadmium oxide, silver-tin oxide, silver-indium oxide), silver graphite, silver molybdenum and silver nickel. (Silver nickels are not to be confused with nickel silvers, a family of copper-nickel-zinc alloys which, interestingly enough, contain no silver.) Table 2 shows how the hardness and conductivity of these composites vary with the amount of silver present. (Data taken from the ASM Handbook Volume 2.)
Silver composite materials are used in make and break contacts operating under high current and/or voltage. These materials are more resistant to damage from electrical arcing and contact welding, which may occur under these loading characteristics.
One example is our iON EV™ clad material. iON EV clad is a proprietary silver-based alloy that combines the reliable qualities of each metal to create a drop-in replacement for the pure silver often used in EV charger connections.
Thanks for joining me for another edition of In Our Element. For ongoing industry updates, connect with me on LinkedIn.