
At Materion, we work with many proprietary processes for creating and customizing metals. Some of the most basic stages involve applying heat to metal to encourage it to take on certain characteristics, like finer grain size or increased conductivities. For example, copper-beryllium alloys are present in a variety of markets and applications and the versatility that variety conveys is due to heat treating. Understanding the science behind heat treating is essential to appreciating the value it adds to our products.
The most powerful strengthening mechanisms are those which rely on thermal treatments. Before or after forming, a heat treatment improves the finished product's strength. Metals that can be strengthened by heat treatments after fabrication offer the best combination of strength and manufacturing capabilities. They will have good to excellent formability prior to thermal strengthening and good to excellent strength after.
Thermal treatments rely on the ability of the atoms in the crystals to diffuse through each other. There are two mechanisms that make this possible. The first is by two atoms switching places, as shown in Figure 1. This requires some additional thermal energy, and rarely happens at room temperature. The second mechanism is by vacancy diffusion. Vacancies are essentially holes in the crystalline lattice. Any neighboring atom can jump into the vacancy. This essentially allows the vacancy to travel through the crystal. Since the order of the atoms in the crystal changes, this provides a convenient means for the atoms to mix. As temperature increases, so does the diffusion rate.
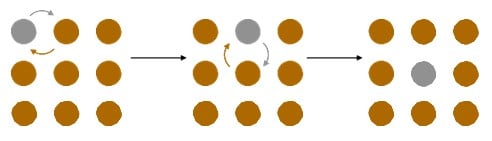
Figure 1. Diffusion of Atoms by Position Exchange

Figure 2. Vacancy Diffusion
Thermal strengthening mechanisms in copper alloys start with solution annealing. The metal is heated to a temperature high enough to cause all the alloying elements to diffuse evenly into the copper. The amount of solute diffused into the copper will be greater than the solubility limit at room temperature. This is like adding sugar to coffee. More sugar can be dissolved into hot coffee than into cold coffee. If a saturated hot coffee-sugar solution is allowed to cool, the excess sugar will precipitate out of the coffee onto the bottom of the cup.
Some alloys are dispersion hardened. This means that as the metal cools from its saturated solution annealed state, fine particles of the alloying elements (usually containing iron or cobalt) precipitate out of the copper. An even distribution of fine particles inside the grains creates an effective means of stopping dislocations. These particles will have a slightly different composition than the rest of the metal. This is basically an enhanced solid solution strengthening mechanism.
Certain other copper alloys such as aluminum bronzes may be quench hardened. Quenching is a rapid cooling of the annealed material. Since there is more alloying element diffused into the copper than the copper can hold at room temperature (super-saturation), the crystalline lattices of the grains are highly distorted. The dislocations are essentially trapped with nowhere to go. This produces an extremely hard, strong, and brittle material. Note that this means the metal is unworkable. The material may then be reheated to a temperature high enough to cause atomic diffusion, but too low to cause annealing and recrystallization. This tempering heat treatment will allow some more stable compounds to precipitate out of the solution. This will improve the ductility of the material, while keeping some of the extra strength.
Precipitation age-hardened alloys, like copper beryllium or copper titanium, also rely on quenching the metal to create a super-saturated solution at room temperature. Unlike a typical quench-hardened alloy, the strength is fairly low and the ductility is fairly high. This permits the metal to be formed. After forming, the metal is heated to a temperature high enough to allow atomic diffusion but still well below the annealing temperature. Fine crystals of a different phase (crystalline structure) will precipitate out of the solution. These precipitates will put strain on the crystalline lattice (see Figure 3), stopping dislocations not only at the precipitate itself but also in the surrounding area. These alloys can attain the highest strengths of all copper alloys.
Precipitation age-hardenable alloys can also be provided in the mill-hardened condition. This means that the material will have been age hardened by the producer before it reaches the stamper. Since the material must still be formed, the mill hardened alloys will not be as strong as the age-hardenable alloys. Each alloy will have its own combination of strength and formability characteristics.
Spinodal decomposition is another thermal strengthening mechanism similar to precipitation age hardening. Spinodal alloys, like ToughMet® copper-nickel-tin, are quenched from the annealed state and can be worked before aging like the precipitation-hardened alloys. After the metal is formed, it is heated at a temperature below the solution annealing temperature. Here, the single phase material will spontaneously decompose into alternating areas of two chemically different but structurally identical phases (see Figure 4). Dislocations are then stopped at the boundaries between the two different phases within each grain.
Thermal strengthening, or heat treatment, is the most effective means of increasing the strength of a metal. When used in combination with cold work and grain size refinement, just about any combination of strength and ductility can be achieved.
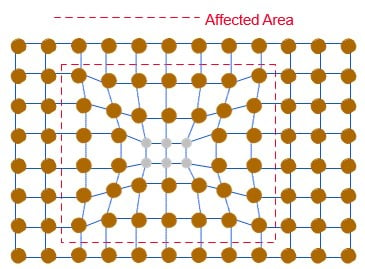
Figure 3. Representation of Precipitation Age Hardening
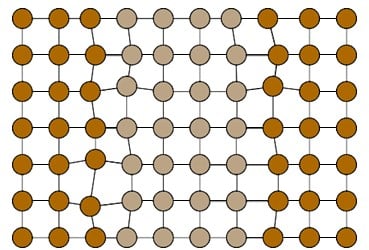
Figure 4. Representation of Spinodal Decomposition
Thanks for joining us for another edition of In Our Element. For ongoing industry updates, connect with us on LinkedIn.