Approximately five thousand years ago, early humans discovered they could make a strong, tough metal by mixing copper and tin together. They had created the world’s first alloy (a mixture of two or more metals). Unaware at the time, they were taking advantage of an important strengthening mechanism, solid solution hardening. The Bronze Age therefore became the dawn of metallurgy.
Solid solution hardening is simply the act of dissolving one metal into another, similar to dissolving sugar into coffee. This is done during casting, when all the metals involved are in liquid form. For electrical connectors, copper is usually the main ingredient and is said to be the solvent, similar to the coffee in the above example. Other elements, playing the role of the sugar, to be added to the copper are known as the solutes.
There is a limit to the amount of solute that can be dissolved in to the solvent. This is known as the solubility limit. For example, coffee will only dissolve so much sugar before the excess settles on the bottom. However, raising the temperature of the solvent can often increase the solubility limit. There are several thermal strengthening methods that depend on having excess solute cast into the material and frozen into place when the mixture cools.
Plastic deformation (permanent set) of contact materials comes from the movement of dislocations throughout crystalline lattices that comprise each grain. Figure 1 shows how dislocations can move easily throughout an unalloyed metal. When other elements are dissolved into the copper, they help to impede the movement of dislocations. This imparts extra strength to the material.
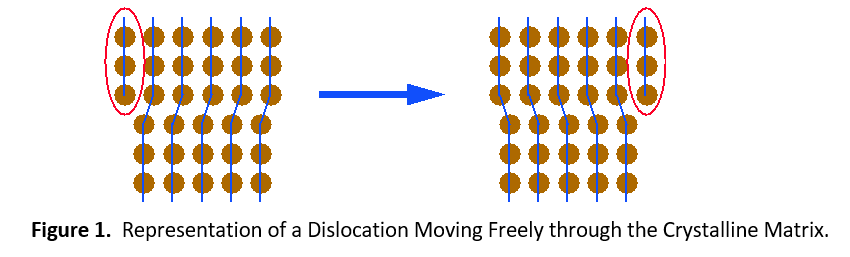
There are two types of solid solutions. The first is a substitutional solution, such as tin in bronze. In this case, atoms of the solute material replace atoms of the solvent material in the crystal lattice (see Figure 1). Since the solute and solvent atoms are different sizes, they interrupt the regularity of the crystal lattice. Dislocations cannot easily move around this interruption. It will take a much higher stress level or temperature to enable the dislocation to move again.
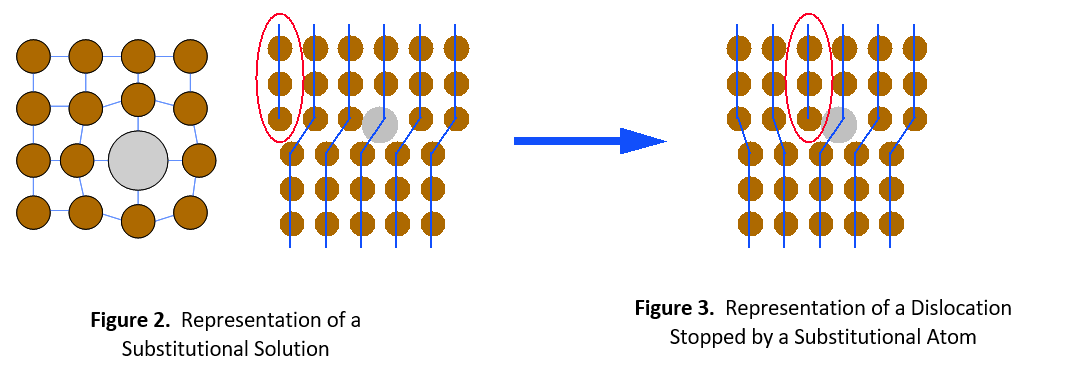
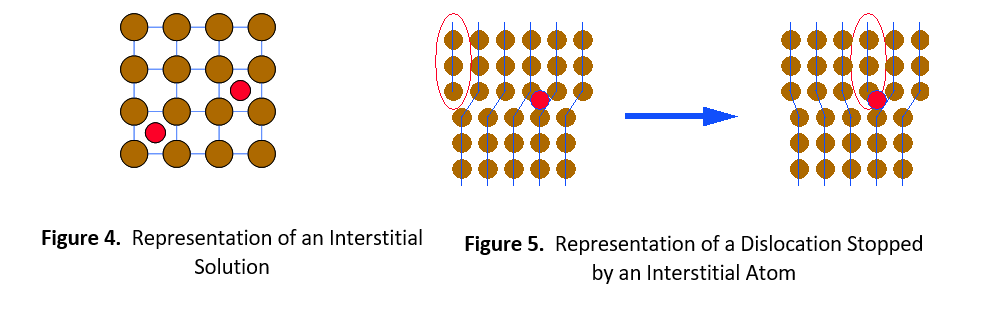
The second type of solid solution is called an interstitial solution, such as carbon in steel. In this case, solute atoms are small enough to fit into spaces (interstices) between the solvent atoms in the crystal lattice (see Figure 4). Once again, the alloying element catches the dislocation and prevents it from moving further. It then requires greater stress or thermal energy for the dislocation to move around the impeding atom.
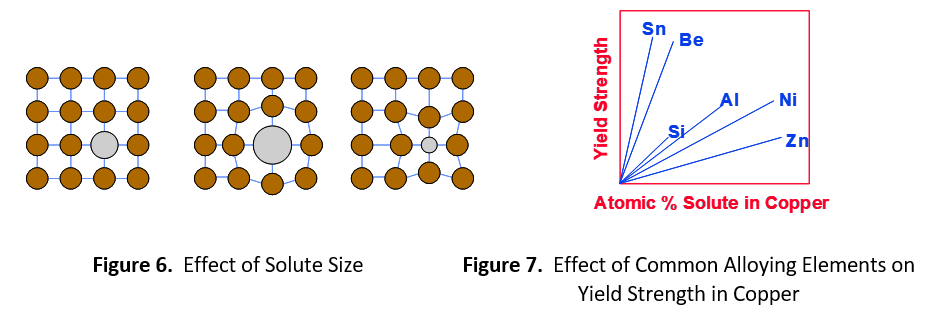
The degree of strength imparted by the alloying element depends on the relative difference in size between the solute and solvent. Figure 6 shows a large difference in size creates more distortion of the crystalline lattice. This extra distortion further impedes the progress of dislocations, resulting in higher strength. Figure 7 shows several common alloying elements used in copper. Based on atomic mass, zinc and copper atoms are nearly the same size, 65.4 and 63.5, respectively. Zinc gives the lowest return on strength. Nickel (58.7) is also very close in size to copper, but not as close as zinc. Aluminum (27.0) and silicon (28.1) are both substantially smaller than copper and give much greater return. Beryllium (9.0) is about 1/7 the size of copper and tin (118.7) is nearly double the size. These elements give the highest return on strength.
Solution hardening, or alloying, is a powerful method to improve the strength of a material. However, there are other equally important methods of improving strength. Various alloying elements are also used in different combinations in efforts to vary other important properties, such as conductivity, elastic modulus, ductility, and cost. All properties of a material must be scrutinized by the designer before a material choice can be made.
Thanks for joining me for another edition of In Our Element. For ongoing industry updates, connect with me on LinkedIn.