Galvanic corrosion can occur when two or more dissimilar metals contact each other in an electrolytic environment such as seawater, chemical processing or in automotive fasteners and connectors exposed to road salt spray.
Two dissimilar metals, alloys or a combination of each in contact with each other in a conductive environment develop a potential difference and make up a galvanic couple. This potential difference is the driving force for one within the couple to oxidize. In this case, the oxidation of the metal or alloy is known as galvanic corrosion. The extent of the corrosion is affected by the environment, the difference in their potentials, the ratio of the cathodic to the anodic areas, and the distance from the junction between the couple.
An electrochemical reaction will occur in any aqueous environment provided four criteria are met. The criteria are: (1) Something must oxidize, which is known as the anode; (2) something else must reduce, which is known as the cathode; (3) both surfaces must be immersed in the same aqueous environment capable of carrying charge, and; (4) there must be an electrically conductive pathway present between the two surfaces outside of the aqueous environment, allowing for the transport of electron(s). When all four criteria exist, the anode, which can be either a metal or alloy, undergoes oxidation (corrosion).
The role of anode or cathode for a given couple will change as the members of the couple change according to the potentials with respect to one another. The metal with the more negative reduction potential will spontaneously undergo oxidation. The reduction potentials are measured against a standard hydrogen electrode, (SHE), whose potential is assigned the value of 0.00 V at 25°C, 1 atm hydrogen fugacity and unit activity of hydrogen ion. A series of metals in a 1 Molar solution of their own ions measured against the SHE is known as the EMF (ElectroMotive Force) series.
Use of these potentials can determine which of two metals will spontaneously assume the roles of anode and cathode. However, it is rare that two metals are in contact with each other in a solution of their own ions. In addition, alloy potentials are not listed in an EMF series. For these reasons, a more practical tool for determining which metal alloy will corrode (when in contact with each other in a conductive environment) is the galvanic series.
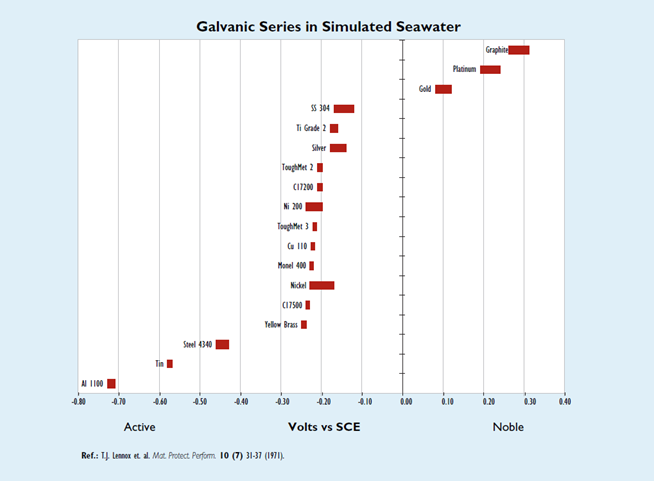
A galvanic series is a list of metals and alloys arranged according to their corrosion potentials as measured in a given environment and presented in tabular or graphical form. Although a galvanic series is most useful for predicting the active metal or alloy in the environment represented in the series, the galvanic series constructed in seawater is the most widely used because it can be applied with caution to similar environments such as other natural waters and uncontaminated atmospheres.
A galvanic series is a tool for predicting which one of two dissimilar metals (and/or alloys) will undergo an accelerated corrosion while in contact with each other in a conductive environment. Since galvanic corrosion is an electrochemical reaction, the four criteria mentioned above still must be met for corrosion to occur. The metal or alloy possessing the more negative (active) potential tends to suffer accelerated corrosion, while the metal or alloy possessing the more positive (noble) potential tends to remain practically unaffected.
Although galvanic series cannot predict corrosion rates, the rate does depend on four factors, one of which is the potential difference that can be estimated from the galvanic series. The first and most significant factor to consider is the environment. Sometimes the potential can reverse for a couple in different environments. This is the reason it is always favored to construct a galvanic series in the environment of interest.
The second factor contributing to the corrosion rate is the potential difference between the couple. The farther apart from each other the two metals or alloys are in the table (i.e., the greater the potential difference between them), the more severe the attack will be on the active metal or alloy.
The third factor deals with the ratio of the cathodic to anodic areas. A particularly unfavorable condition to set up between the couple is one in which there is a large cathode to small anode area ratio because, for a given current flow, the current density will be larger for the smaller anode area; hence the corrosion will proceed at a greater rate. The fourth factor is the distance from the junction between the couple. The greatest amount of corrosion will occur at the junction.
The following graph is for a galvanic series in simulated seawater. Unless noted, the data was acquired using the specifications set forth by ASTM 82 (Standard Guide for Development and Use of a Galvanic Series for Electrochemical Measurements in Corrosion Testing). The seawater was prepared according to the guidelines in ASTM D1141 (Standard Specifications for Substitute Ocean Water) except for the addition of SrCl2.
Find out more about our materials with high corrosion resistance, such as ToughMet alloy and ULTRA 76 plus tantalum alloy.
Thanks for joining us for another edition of In Our Element. For ongoing industry updates, connect with us on LinkedIn.